I have finalised the demo for the ICH-GCP E6 R3 refresher course. Overall, I liked the content and the interface. I also want to thank Whitehall Train...
About
Escrito por un experto con más de 30 años de experiencia hasta el nivel de Director de Investigación en un importante grupo farmacéutico, este curso de capacitación en GCP cubre las pautas internacionales ICH-GCP (E6-R3) y cumple con los requisitos de capacitación para participar en ensayos clínicos internacionales.
Course Syllabus
- La historia de GCP: Parte 1
- La historia de GCP: Parte 2
- La historia de GCP: Parte 3
- La historia de GCP: Parte 4
- ¿Qué es GCP?
- Los principios de ICH GCP: Parte 1
- Los principios de ICH GCP: Parte 2
- Puntos de aprendizaje adicionales
- Documentación y control de versiones
- Control de calidad (QA)
- Recursos clave: Parte 1
- Recursos clave: Parte 2
- Introducción: Parte 1
- Introducción: Parte 2
- Introducción: Parte 3
- Introducción: Parte 4
- Introducción: Parte 5
- Introducción: Parte 6
- Introducción: Parte 7
- Introducción: Parte 8
- Introducción: Parte 9
- Responsabilidades de la Autoridad Reguladora
- Responsabilidades del IEC
- Formularios de Consentimiento Informado del Sujeto (ICF) Parte 1
- Formularios de Consentimiento Informado del Sujeto (ICF): Parte 2
- Composición, Funciones, Operaciones, Procedimientos y Registros
- Interacciones del IEC con Patrocinadores e Investigadores
- Introducción
- Responsabilidades del investigador
- Cualificaciones y acuerdos del investigador
- Recursos adecuados
- Atención médica a los sujetos del ensayo: Parte 1
- Atención médica a los sujetos del ensayo: Parte 2
- Comunicación con el Comité de Ética de Investigación (CRI)/Comité de Ética de Investigación (CEI)
- Cumplimiento del protocolo
- Medicamentos en investigación
- Procedimientos de aleatorización y desenmascaramiento
- Consentimiento informado: Introducción
- Consentimiento informado: Discusión sobre el consentimiento
- Consentimiento informado: Sujetos que no saben leer ni escribir
- Consentimiento informado: Menores y sujetos con discapacidad mental
- Consentimiento informado: Sujetos con discapacidad mental
- Consentimiento informado: Actualización del consentimiento
- Documentos e informes: Introducción
- Documentos e informes: Archivos del centro de estudio
- Documentos e informes: Actualizaciones y cambios
- Documentos e informes: Documentos fuente
- Documentos e informes: Información financiera Información
- Documentos e informes: Formulario de consentimiento informado
- Documentos e informes: Registro de casos
- Documentos e informes: Registro de datos de los sujetos
- Terminación anticipada o suspensión de un estudio
- Informes de progreso e informes finales del investigador
- Responsabilidades del investigador
- Introducción: Parte 1
- Introducción: Parte 2
- Introducción: Parte 3
- Gestión de la Calidad: Parte 1
- Gestión de la Calidad: Parte 2
- Gestión de la Calidad - Parte 3
- QA y QC (Garantía y Control de Calidad): Introducción
- QA y QC (Garantía y Control de Calidad): Procedimientos Operativos Estándar
- QA y QC (Garantía y Control de Calidad): Acuerdos y Contratos
- Organizaciones de Investigación por Contrato
- Diseño del Estudio
- Gestión del Estudio: Introducción
- Gestión del Estudio: Gestión de Datos
- Gestión del Estudio: Datos Electrónicos
- Gestión del Estudio: Retención de Registros
- Selección del Investigador: Introducción
- Selección del Investigador: Autorizaciones
- Selección del Investigador: Responsabilidades
- Selección del Investigador: Remuneración
- Financiación
- Notificación/Presentación a las Autoridades Reguladoras
- Confirmación de la Revisión del Comité de Ética de Investigación (CRI)/Comité de Ética de Investigación (CEI)
- Información del IMP
- Fabricación, Envasado, Etiquetado y Codificación de Productos en Investigación: Parte 1
- Fabricación, Envasado, Etiquetado y Codificación de Productos en Investigación: Parte 2
- Suministro y Manejo de Productos en Investigación
- Acceso a los Registros
- Gestión de Datos I
- Gestión de Datos II
- Gestión de Datos III
- Gestión de Datos IV
- Gestión de Datos V
- Gestión de Datos VI
- Programación Estadística y Análisis de Datos I
- Programación Estadística y Análisis de Datos II
- Retención y Archivo de Datos
- Auditoría e Inspección
- Incumplimiento
- Terminación o Suspensión Anticipada de un Ensayo Clínico: Parte 1
- Terminación o Suspensión Anticipada de un Ensayo Clínico: Parte 2
- Informes de Estudios Clínicos
- Estudios Multicéntricos
- Introducción
- Gobernanza de Datos - Parte 1
- Gobernanza de Datos - Parte 2
- Mantenimiento de la Ciega
- Ciclo de Vida de los Datos I
- Ciclo de Vida de los Datos II
- Ciclo de Vida de los Datos III
- Ciclo de Vida de los Datos IV
- Sistemas de Información I
- Sistemas de Información II
- Sistemas de Información III
- Sistemas de Información IV
- Sistemas de Información V
- Sistemas de Información VI
- Sistemas de Información VII
- Introducción
- Monitorización
- La visita de monitorización: Parte 1
- La visita de monitorización: Parte 2
- Verificación del PMI
- Cumplimiento del protocolo, las enmiendas, los procedimientos operativos estándar y las directrices
- Verificación del consentimiento informado
- El formulario de registro de caso (CRF) y los documentos fuente
- Verificación de los datos del sujeto
- Cierre de la visita de monitorización
- El informe y el plan de monitorización
- Gestión de la calidad: monitorización centralizada
- Fraude y mala conducta: Parte 1
- Fraude y mala conducta: Parte 2
- Introducción
- Eventos adversos, reacciones adversas a medicamentos y reacciones adversas graves (SUSAR)
- Eventos adversos graves (SUSAR)
- Eventos adversos de especial preocupación
- Informes periódicos de seguridad
- Introducción
- Estructura y contenido del protocolo: Parte 1
- Estructura y contenido del protocolo: Parte 2
- Estructura y contenido del protocolo: Parte 3
- Introducción
- Estructura y contenido del folleto del investigador
- Presentaciones
- Presentación
- Documentos que deben presentarse antes del estudio
- Documentos que deben presentarse después del estudio
- Glosario y abreviaturas
- Documentos de orientación de la UE
- Documentos de orientación de la ICH
- Documentos de orientación de la FDA de EE. UU.
- Lista de autoridades competentes mundiales
Our Certified Customers
Learner Rating & Reviews
Frequently Asked Questions
Good Clinical Practice (GCP) training is a vital educational programme designed to arm researchers and clinical trial professionals with essential knowledge of ethical and scientific standards. These standards are crucial for executing high-quality clinical trials. The training encompasses the globally acknowledged guidelines set forth by the International Council for Harmonisation (ICH).
The key objectives of GCP training include:
- Safeguarding the rights, safety, and welfare of human participants
- Upholding the accuracy and reliability of data collected during clinical trials
- Fostering uniform, superior practices across all facets of clinical research
Whitehall Training's Good Clinical Practice Course thoroughly explores these critical areas. Our comprehensive programme equips learners with a robust understanding of GCP principles and their practical implementation in real-world clinical research scenarios. By completing this course, participants gain the necessary skills to conduct clinical trials that meet the highest standards of ethical and scientific rigour.
Absolutely. For those involved in clinical trials, GCP certification isn’t just valuable—it’s essential. But even if you’re not directly participating in trials, obtaining GCP certification offers numerous benefits:
- It demonstrates your commitment to upholding global research standards
- Your research gains added credibility and quality assurance
- You’ll be better equipped to safeguard the rights and wellbeing of study participants
- Your career opportunities in the clinical research sector may expand significantly
Our Whitehall Training Good Clinical Practice Course goes beyond mere certification. We provide you with practical, hands-on knowledge that you can immediately apply in your professional endeavours. By mastering GCP principles through our course, you’ll be well-prepared to navigate the complexities of clinical research with confidence and expertise.
Good Clinical Practice (GCP) certification is crucial for a broad spectrum of individuals working in clinical research:
- Lead Researchers and Co-investigators: These are the primary and supporting scientists responsible for overseeing and conducting trials at research facilities.
- On-site Trial Team: This includes study coordinators, research nurses, and other personnel involved in the day-to-day management of clinical trials.
- Trial Sponsors and Research Organisations: Professionals who manage the overall planning, commencement, and documentation of clinical studies.
- Regulatory Bodies: Officials tasked with supervising and assessing trial compliance with established standards.
- Ethics Committees: Groups responsible for reviewing and approving proposed trial protocols to ensure ethical conduct.
- University and Research Centre Staff: Individuals ensuring that institutional research aligns with international benchmarks.
- Researchers Funded by National Health Organisations: All scientists and support staff engaged in publicly funded clinical trials.
Our comprehensive GCP course is designed to meet the needs of this varied audience, offering both universal principles and role-specific guidance. Additionally, it serves as an excellent resource for those aiming to enhance their research capabilities and refine their approach to clinical trial operations. The Whitehall Training Good Clinical Practice Course provides tailored content to address the unique requirements of each of these professional groups, ensuring that all participants gain the knowledge necessary for their specific roles in the clinical research process.
Our Good Clinical Practice (GCP) course is designed to provide comprehensive coverage of the ICH-GCP (E6-R3) international guidelines, ensuring you meet the necessary training requirements for participation in international clinical trials. We offer this course in multiple languages to cater to a diverse audience.
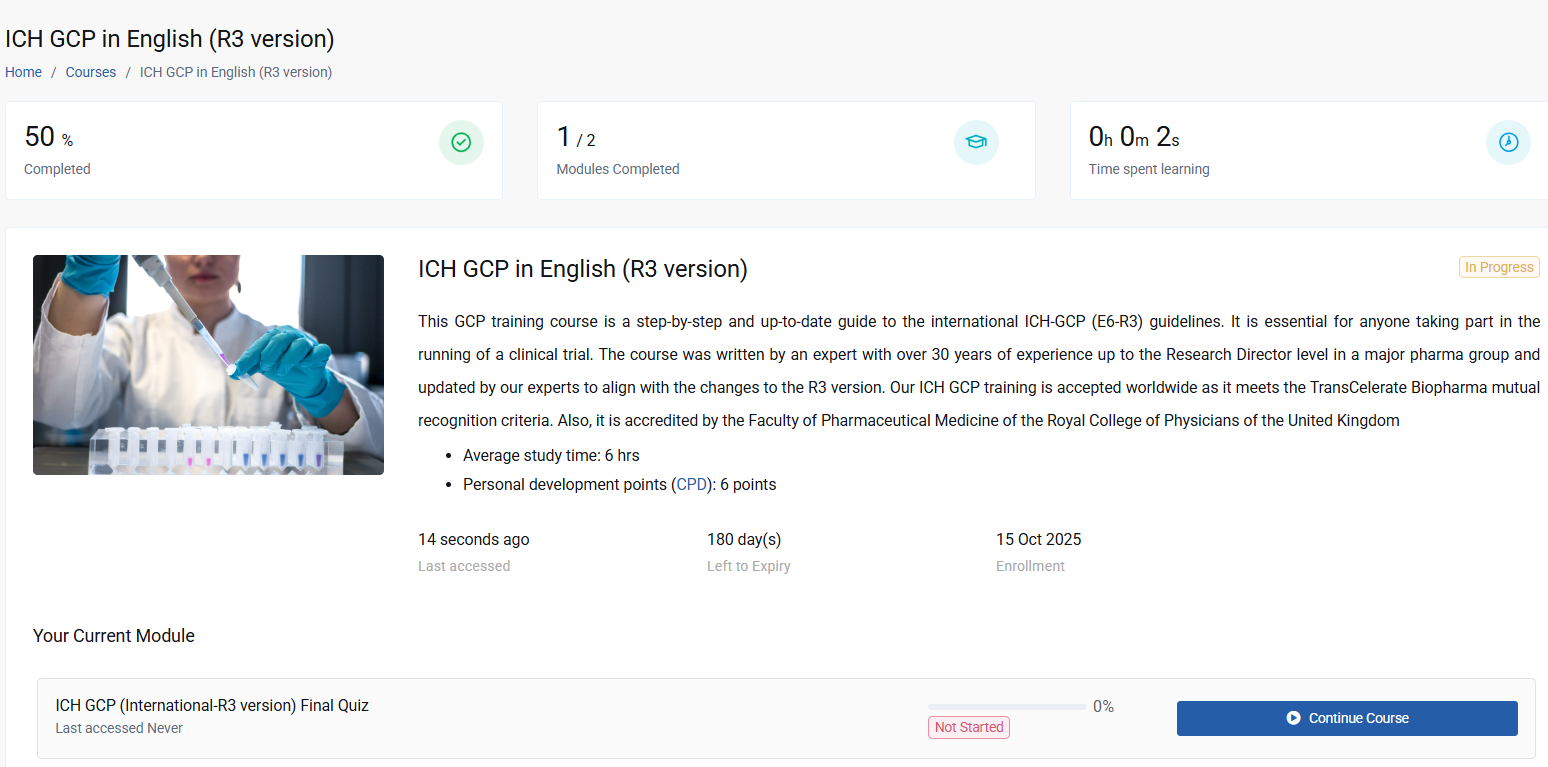
This course serves as a detailed, step-by-step guide to the most recent ICH-GCP (E6-R3) guidelines, making it an indispensable resource for anyone involved in clinical trial management. The content has been meticulously crafted by an industry veteran with over three decades of experience, including a tenure as Research Director at a leading pharmaceutical company.
We’re proud to offer a course that’s accredited by the Faculty of Pharmaceutical Medicine of the Royal College of Physicians of the United Kingdom. This accreditation underscores the quality and relevance of our training material. To accommodate our global learners, we’ve made the course available in ten languages: English, German, Bulgarian, French, Italian, Japanese, Polish, Portuguese, Russian, and Spanish. Additionally, we’ve developed region-specific versions tailored to the regulatory frameworks of Australia, the UK, the US, France, Germany, and Latin America.
Our course stands out for its user-friendly design and clear visual presentation. This format allows for easy navigation and reference to the ICH-GCP E6 document. Drawing from the author’s extensive industry experience, the course offers practical insights into the application of GCP principles.
Upon completion, participants can earn 6 Continuing Professional Development (CPD) points, further enhancing the value of this training programme.
Indeed, our GCP course has received certification from the Faculty of Pharmaceutical Medicine at the Royal College of Physicians. This prestigious body, established in 1989, is renowned for its role in setting stringent research standards and serves as the professional membership organisation for pharmaceutical physicians across the United Kingdom.
The certification bestowed upon our course is a testament to its quality and relevance. It signifies that our programme meets exacting industry and academic criteria, providing you with a qualification that is widely acknowledged and respected within the field. By completing this certified course, you can be confident that you’re receiving training that aligns with the most current and rigorous standards in pharmaceutical research and practice.
The price of GCP certification can fluctuate based on several key factors:
- Endorsement: Has the course received approval from official bodies?
- Validity: Does the training meet ICH standards, allowing researchers to engage in global clinical studies?
- Content quality: Is the material current and authored by an industry expert?
- Duration of access: For how long can students utilise the course materials?
Our Whitehall Training GCP course is priced at £79, reflecting its high value across these areas:
- Endorsement: The Royal College of Physicians has accredited our course, awarding it 6 CPD points.
- Validity: Upon completion, researchers are equipped to participate in clinical trials, in line with ICH E6(R3) guidelines.
- Content quality: Lucy Parker, our GCP specialist with over ten years’ experience leading research at major institutions like the NHS, has crafted the course content.
- Duration of access: We support ongoing professional development by offering unlimited access to course materials.
For group purchases, we offer a 10% discount when buying 5 licences at checkout. If you’re interested in larger group discounts, please reach out to our team for a tailored quote.