44 posts
| Featured Image | Title | Category | Published |
|---|---|---|---|
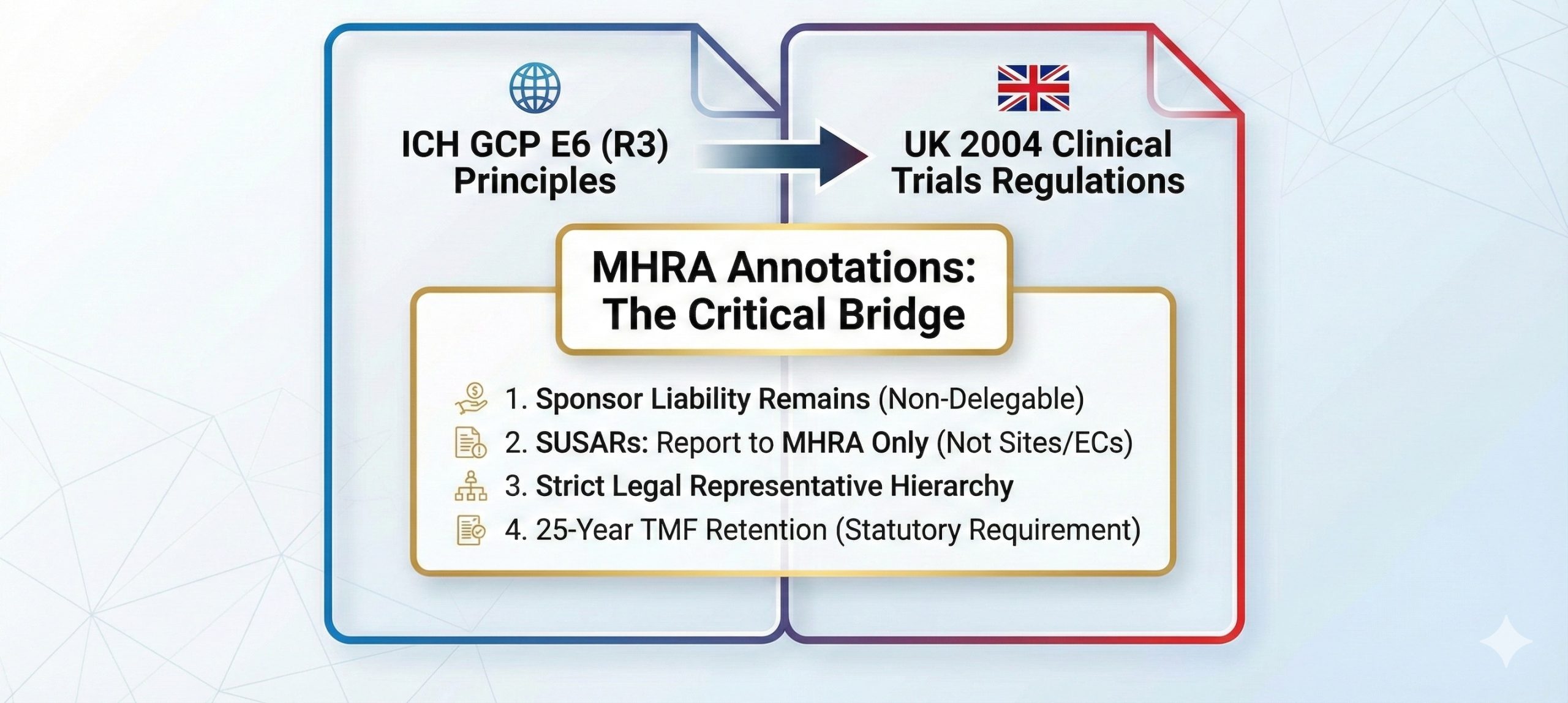
| ICH E6(R3) in the UK Regulatory Landscape | GCP | January 15, 2026 | |
| UK Clinical Trials: The “ICH E6 (R3) + 2004 Regulations” Reality Check | GCP | January 14, 2026 | |
| Beyond the Guidelines: 5 Things You Must Know About FDA Clinical Trial Oversight (ICH GCP E6 R3) | GCP | January 7, 2026 | |
| FDA’s Paradigm Shift: Real-World Evidence in Medical Device Regulation and the Transformation of Clinical Research Workforce Development | EU MDR | January 7, 2026 | |
| From Notice to Closure: Your 5-Point Guide to Acing a GCP Inspection | GCP | January 5, 2026 | |
| 5 Core Principles of Flawless Clinical Research | GCP | January 3, 2026 | |
| Protocol Amendments: When is Re-Consenting Actually Mandatory? | GCP | January 3, 2026 | |
| Why Clinical Data Governance is the Heart of the New ICH GCP E6 (R3) | GCP | December 31, 2025 | |
| 5 Truths About Data Governance in Modern Clinical Research | GCP | December 30, 2025 | |
| 6 Truths Behind the Rules That Safeguard Every Clinical Trial | GCP | December 29, 2025 | |
| 5 Ways Europe’s Medical Device Rules Are Reshaping Healthcare and Patient Safety | EU MDR | December 14, 2025 | |
| Whitehall Training has been officially designated as a RAPS Recertification Approved Provider by the Regulatory Affairs Professionals Society (RAPS) | WhiteHall | December 3, 2025 | |
| Stay compliant with the latest ICH GCP update | GCP | November 26, 2025 | |
| The future of ClinicalTrials is digital | GCP | November 26, 2025 | |
| How to Get an ICH E6 (R2, R3) Certification in 2025 | GCP | November 26, 2025 | |
| Navigating the Shift: ICH GCP E6 (R3) vs. (R2) and the Future of Clinical Compliance | GCP | November 24, 2025 | |
| Beyond the Microscope: The Surprising Responsibilities of a Clinical Trial Investigator (ICH GCP E6 R3) | GCP | November 23, 2025 | |
| 5 Skills That Make You Employable in Pharmacovigilance | Pharmacovigilance | November 22, 2025 | |
| Expanding Access to GCP E6(R3): Now Available in 10+ Languages (and growing!): As global clinical trials continue to grow, so does the need for high-quality, accessible Good Clinical Practice training | GCP | November 15, 2025 | |
| New Course Launch: ICH GCP E6(R3) for Site Investigators | GCP | November 14, 2025 | |
| New Course Launch: ICH GCP (E6 R3) Refresher Course | GCP | November 12, 2025 | |
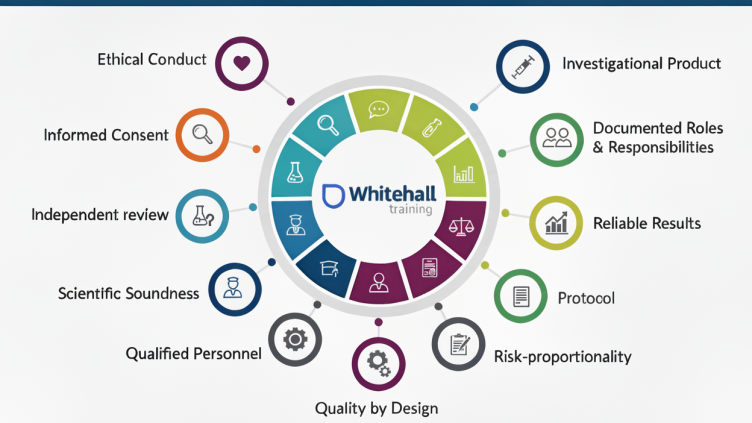
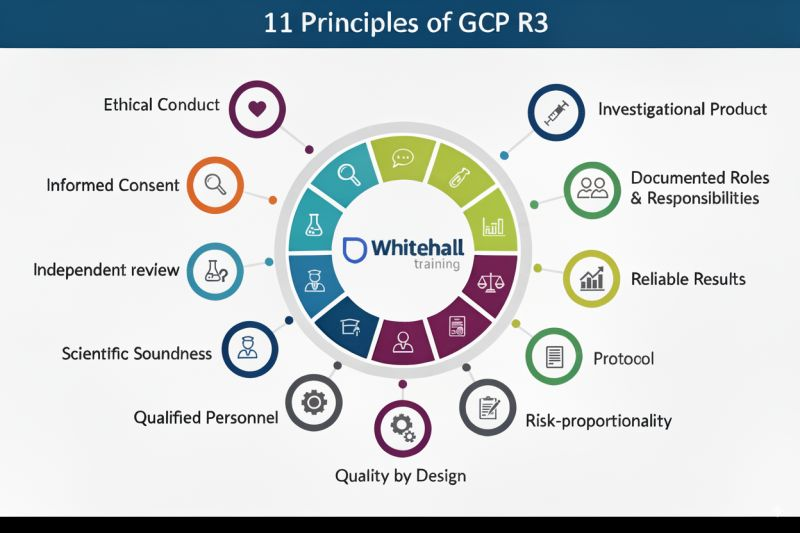
| How to Get an ICH E6(R2,R3) Certification [2025] | GCP | November 11, 2025 | |
| Good Clinical Data Management Practice Guidelines [2025] | GCP | November 10, 2025 | |
| More Than Just the Money: 4 Pillars of a Clinical Trial Sponsor’s Stewardship | GCP | November 9, 2025 | |
| The Unseen Blueprint: 5 Rules That Govern Every Medical Breakthrough (ICH GCP E6 R3) | GCP | November 8, 2025 | |
| The Hidden Rules of Medical Breakthroughs: 5 Truths About Clinical Trials (ICH GCP E6 R3) | GCP | November 5, 2025 | |
| More Than a Brochure: 5 Truths About the Rulebook for Clinical Trials (ICH GCP E6 R3) | GCP | November 4, 2025 | |
| Clinical Trial Protocol is more than a recipe: 5 Truths About the Blueprint for Medical Breakthroughs (ICH GCP E6 R3) | GCP | November 1, 2025 | |
| Beyond the FDA Guidelines: 5 Things You Must Know About Clinical Trial Oversight in ICH GCP E6 R3 | GCP | October 31, 2025 | |
| Beyond Side Effects: 5 Critical Safety Reporting Rules That Protect Patients in Clinical Trials (ICH GCP E6 R3) | GCP | October 30, 2025 | |
| Beyond GCP & GLP: Unpacking the Gold Standard for Clinical Trial Labs via GCLP | WhiteHall | October 29, 2025 | |
| E6(R3) and Real-World Data: Integrating EHRs, Registries, and Digital Health Tools into GCP-Compliant Trials | GCP | October 28, 2025 | |
| Preparing for GCP Inspections Under E6(R3): What Auditors Will Look For | GCP | October 26, 2025 | |
| Global Harmonization of GCP: Comparing E6(R3) Implementation Across Regions | GCP | October 24, 2025 | |
| Ethics Committees and E6(R3): Strengthening Independent Review in a Risk-Based Framework | GCP | October 23, 2025 | |
| Investigator Responsibilities Under E6(R3): Oversight, Delegation, and Training | GCP | October 22, 2025 | |
| Decentralized Trials and E6(R3): Regulatory Expectations and Practical Implementation | GCP | October 21, 2025 | |
| Essential Records and Data Governance: Navigating E6(R3)’s New Documentation Framework | GCP | October 20, 2025 | |
| Informed Consent in the E6(R3) Era: Flexibility, Documentation, and Ethics | GCP | October 10, 2025 | |
| What Are Temporal Relationship Types? 3 Key Insights for 2024 | Pharmacovigilance | September 7, 2024 | |
| How to Develop Initiative: 7 Steps for Proactive Professionals | GCP | September 4, 2024 | |
| FDA Pre-Approval Inspection Guidance 2024: 5 Expert Tips to Succeed | Pharmacovigilance | August 22, 2024 | |
| Good Clinical Practice Jobs List [2025] | GCP | August 1, 2024 | |
| The Good Clinical Practice Guidelines [2025] | GCP | July 31, 2024 |










![How to Get an ICH E6(R2,R3) Certification [2025]](https://wp.whitehalltraining.com/wp-content/uploads/2025/11/68b6be6eb3569e524962630f_good-clinical-practice-11-scaled.jpg)
![Good Clinical Data Management Practice Guidelines [2025]](https://wp.whitehalltraining.com/wp-content/uploads/2025/11/68b6be6eb3569e5249626330_research-ethics_29-scaled.jpg)












![Good Clinical Practice Jobs List [2025]](https://wp.whitehalltraining.com/wp-content/uploads/2025/11/66a7ad41877ee0c9191f34f6_research-ethics_28-scaled.jpg)