I have finalised the demo for the ICH-GCP E6 R3 refresher course. Overall, I liked the content and the interface. I also want to thank Whitehall Train...
About
大手製薬グループの研究ディレクターレベルまでの 30 年以上の経験を持つ専門家によって執筆されたこの GCP トレーニング コースは、ICH-GCP (E6-R3) 国際ガイドラインをカバーし、国際臨床試験に参加するためのトレーニング要件を満たしています。
Course Syllabus
- GCPの歴史:パート1
- GCPの歴史:パート2
- GCPの歴史:パート3
- GCPの歴史:パート4
- GCP とは何ですか?
- ICH GCPの原則:パート1
- ICH GCPの原則:パート2
- 追加の学習ポイント
- ドキュメントとバージョン管理
- 品質保証(QA)
- 主要リソース: パート 1
- 主要リソース:パート2
- はじめに:パート1
- はじめに:パート2
- はじめに:パート3
- はじめに:パート4
- はじめに:パート5
- はじめに:パート6
- はじめに:パート7
- はじめに:パート8
- はじめに:パート9
- 規制当局の責任
- IECの責任
- 被験者インフォームドコンセントフォーム(ICF)パート1
- 被験者インフォームドコンセントフォーム(ICF):パート2
- 構成、機能、操作、手順、記録
- IECとスポンサーおよび研究者との交流
- 導入
- 研究者の責任
- 研究者の資格と同意
- 適切なリソース
- 被験者の医療ケア:パート1
- 被験者の医療ケア:パート2
- IRB/IECとのコミュニケーション
- プロトコルへの準拠
- 治験薬
- ランダム化手順と非盲検化
- インフォームド・コンセント:序論
- インフォームド・コンセント:同意に関する議論
- インフォームド・コンセント:読み書きができない対象者
- インフォームド・コンセント:未成年者および「精神的に無能力」な対象者
- インフォームド・コンセント:無能力者
- インフォームド・コンセント:同意の更新
- 記録と報告書:はじめに
- 記録と報告書:研究サイトファイル
- 記録と報告書:更新と修正
- 記録と報告書:ソース文書
- 記録と報告書:財務情報
- 記録と報告書:ケース記録フォーム
- 記録とレポート:被験者データの記録
- 試験の早期終了または中断
- 研究者による進捗報告書と最終報告書
- 研究者の責任
- はじめに:パート1
- はじめに:パート2
- はじめに:パート3
- 品質管理:パート1
- 品質管理:パート2
- 品質管理パート3
- QAとQC(品質保証と品質管理):はじめに
- QAとQC(品質保証と品質管理):標準操作手順
- QAとQC(品質保証と品質管理):契約と合意
- 契約研究機関
- トライアルデザイン
- 治験管理: はじめに
- 試験管理:データ管理
- 試験管理:電子データ
- 試験管理:記録の保管
- 研究者の選定:はじめに
- 調査員の選択: 権限
- 研究者の選定:責任
- 研究者の選定:報酬
- 融資
- 規制当局への通知/提出
- IRB/IECによる審査の確認
- IMPに関する情報
- 治験薬の製造、包装、ラベル表示、およびコード化:パート1
- 治験薬の製造、包装、ラベル表示、およびコード化:パート2
- 治験薬の供給と取り扱い
- レコードアクセス
- データハンドリングI
- データハンドリングⅡ
- データハンドリングⅢ
- データ処理IV
- データ処理 V
- データ処理VI
- 統計プログラミングとデータ分析I
- 統計プログラミングとデータ分析 II
- 記録の保管と保存
- 監査と検査
- 不遵守
- 裁判の早期終了または中断:パート1
- 裁判の早期終了または中断:パート2
- 臨床試験/研究報告書
- 多施設試験
- 導入
- データガバナンス パート1
- データガバナンスパート2
- 盲人を維持する
- データライフサイクルI
- データライフサイクルII
- データライフサイクルIII
- データライフサイクルIV
- コンピュータ化システムI
- コンピュータ化システムⅡ
- コンピュータ化システムⅢ
- コンピュータ化システムIV
- コンピュータ化システム V
- コンピュータ化システムVI
- コンピュータ化システム VII
- 導入
- モニター
- モニタリング訪問:パート1
- モニタリング訪問:パート2
- IMPの検証
- 議定書、改正、SOP、ガイダンスの遵守
- インフォームド・コンセントの確認
- 症例記録フォーム(CRF)とソース文書
- 被験者データの検証
- モニタリング訪問の終了
- モニタリング報告書と計画
- 品質管理 - 集中監視
- 詐欺と不正行為:パート1
- 詐欺と不正行為:パート2
- 導入
- AE、ADR、SUSAR
- 重篤な有害事象
- スサラス
- 特に注目すべき有害事象
- 定期安全報告書
- 導入
- プロトコルの構造と内容:パート1
- プロトコルの構造と内容:パート2
- プロトコルの構造と内容:パート3
- 導入
- 治験薬概要書の構成と内容
- はじめに
- アーカイブ
- 事前調査に提出する必要がある書類
- 学習後に提出する必要がある書類
- Glossary & Abbreviations
- EU Guidance Documents
- ICH Guidance Documents
- US FDA Guidance Documents
- GCP Course Printouts
- Global Competent Authorities List
Our Certified Customers
Learner Rating & Reviews
Frequently Asked Questions
Good Clinical Practice (GCP) training is an essential educational program that equips researchers and clinical trial professionals with the knowledge of ethical and scientific standards crucial for conducting high-quality clinical trials. This comprehensive training covers the internationally recognized guidelines established by the International Council for Harmonisation (ICH).
The primary goals of GCP training are:
- Ensuring the protection of human subjects' rights, safety, and well-being
- Maintaining the integrity and reliability of clinical trial data
- Promoting consistent, high-quality practices across all aspects of clinical research
Our GCP course delves into these critical areas, providing learners with a solid foundation in the principles and practical applications of Good Clinical Practice.
For researchers interested in participating in clinical trials, GCP certification is a necessity.
For those who are simply interested in improving their understanding of the field, GCP certification is highly valuable due to its:
- Ensures compliance with international standards
- Enhances research credibility and quality
- Protects participant rights and safety
- Improves career prospects in clinical research
Our course not only provides certification but also equips you with practical skills to apply GCP principles effectively in your work.
GCP certification is essential for a wide range of professionals in clinical research:
- Clinical Trial Investigators: Principal investigators and sub-investigators responsible for trial conduct at research sites.
- Clinical Trial Staff: Including research coordinators, study nurses, and other site personnel involved in trial management.
- Sponsors and Contract Research Organizations (CROs): Those overseeing trial planning, initiation, and reporting.
- Regulatory Authorities: Officials who monitor and evaluate trial compliance.
- Institutional Review Boards (IRBs) and Ethics Committees: Members reviewing and approving trial protocols.
- Academic and Research Institution Staff: Ensuring adherence to international standards in institutional research.
- NIH-Funded Researchers: All investigators and staff involved in NIH-funded clinical trials.
Our course caters to this diverse audience, providing role-specific insights alongside core GCP principles. The course is also valuable for anyone looking to upskill their research abilities and improve their clinical trials operations.
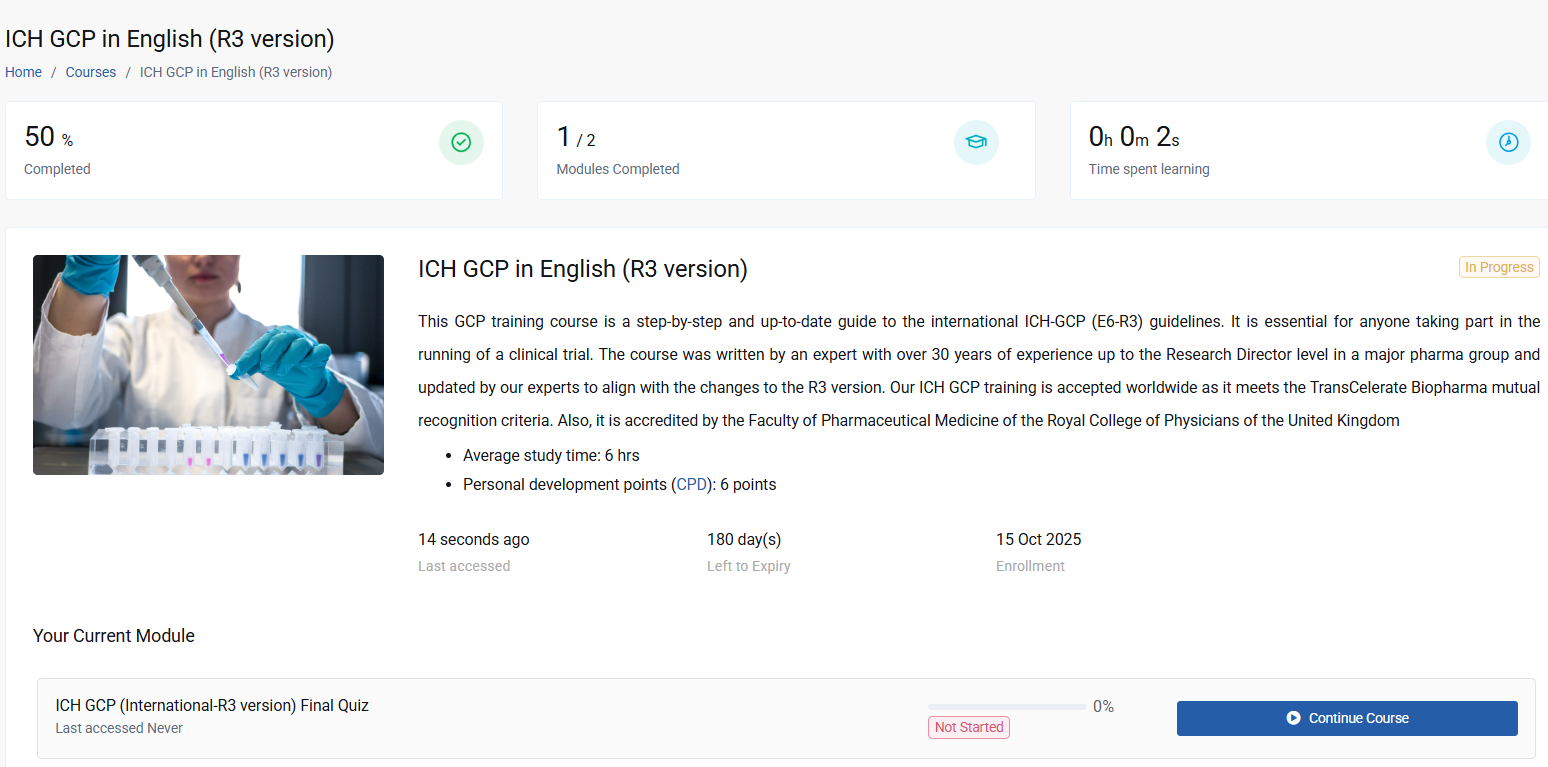
This GCP training course covers the ICH-GCP (E6-R3) international guidelines and meets the training requirement for you to participate in international clinical trials. Multiple language versions are available. This GCP training course is a step-by-step and up-to-date guide to the international ICH-GCP (E6-R3) guidelines. It is essential for anyone taking part in the running of a clinical trial.
The course was written by an expert with over 30 years of experience up to the Research Director level in a major pharma group. Our ICH GCP training is accepted worldwide as it meets the TransCelerate Biopharma mutual recognition criteria. Also, it is accredited by the Faculty of Pharmaceutical Medicine of the Royal College of Physicians of the United Kingdom. Apart from English, the course is available in German, Bulgarian, French, Italian, Japanese, Polish, Portuguese, Russian and Spanish. We also produce regional versions of this course tailored to the specific regulatory frameworks in Australia, the UK, the US, France, Germany and Latin America.
This course features a clear and visually appealing format, allowing for easy cross-referencing to the ICH-GCP E6 document. It offers valuable insights into the practical application of Good Clinical Practice (GCP) based on the author's extensive experience. Furthermore, it is accredited by the Faculty of Pharmaceutical Medicine of the Royal College of Physicians of the United Kingdom and provides participants with the opportunity to earn 6 CPD points.
Yes, our GCP course is accredited by two industry-leading organisations:
- TransCelerate Biopharma Inc.: A nonprofit organisation collaborating with 20 major pharmaceutical companies. Their mutual recognition program is considered the gold standard in the field of clinical practice.
- The Faculty of Pharmaceutical Medicine at the Royal College of Physicians: The professional membership body for pharmaceutical physicians in the UK, known for setting rigorous standards for research since 1989.
These accreditations ensure our course meets the highest industry and academic standards, offering you a widely recognized certification.
Costs vary depending on the following factors:
- Accreditation: Is the course approved by official organisations, like TransCelerate?)
- Certification: Does the course meet the ICH requirements that allows researchers to participate in international clinical trials?
- Quality of content: Is the course up-to-date, and written by an expert?
- Access: How long are learners able to access the course?
Whitehall Training’s GCP course is £79 due to its:
- Accreditation: It is TransCelerate approved, and accredited by the Royal College of Physicians with 6 CPD points.
- Certification: The course enables users to participate in clinical trials, following the ICH E6(R3) guidelines.
- Quality of content: Our course is written by our Good Clinical Practice expert, Lucy Parker, who has over a decade of experience directing research across large research institutes such as the NHS.
- Access: To support the lifelong learning of our researchers, we provide lifetime access to our course resources.
Buying for a team? We offer 10% off orders of 5 licences at checkout. For discounts on larger orders, please get in touch with our team.